-

Precision medicine and complex care environments. Dynamics in market access and reimbursement.
The health technology space has never been that exciting… and challenging at the same time. Gene therapies, microbiome products, digital therapeutics – 2023 was full of exciting market entries from the emerging healthtech field.
-
Announcement of UK IDAP Pilot for medical devices – What does it mean for reimbursement?
The Innovative Devices Access Pathway (IDAP) Pilot is a first step to providing a faster and supported route to regulatory approval, access and adoption of innovative devices. But what precisely does this mean ultimately for access and reimbursement?
-
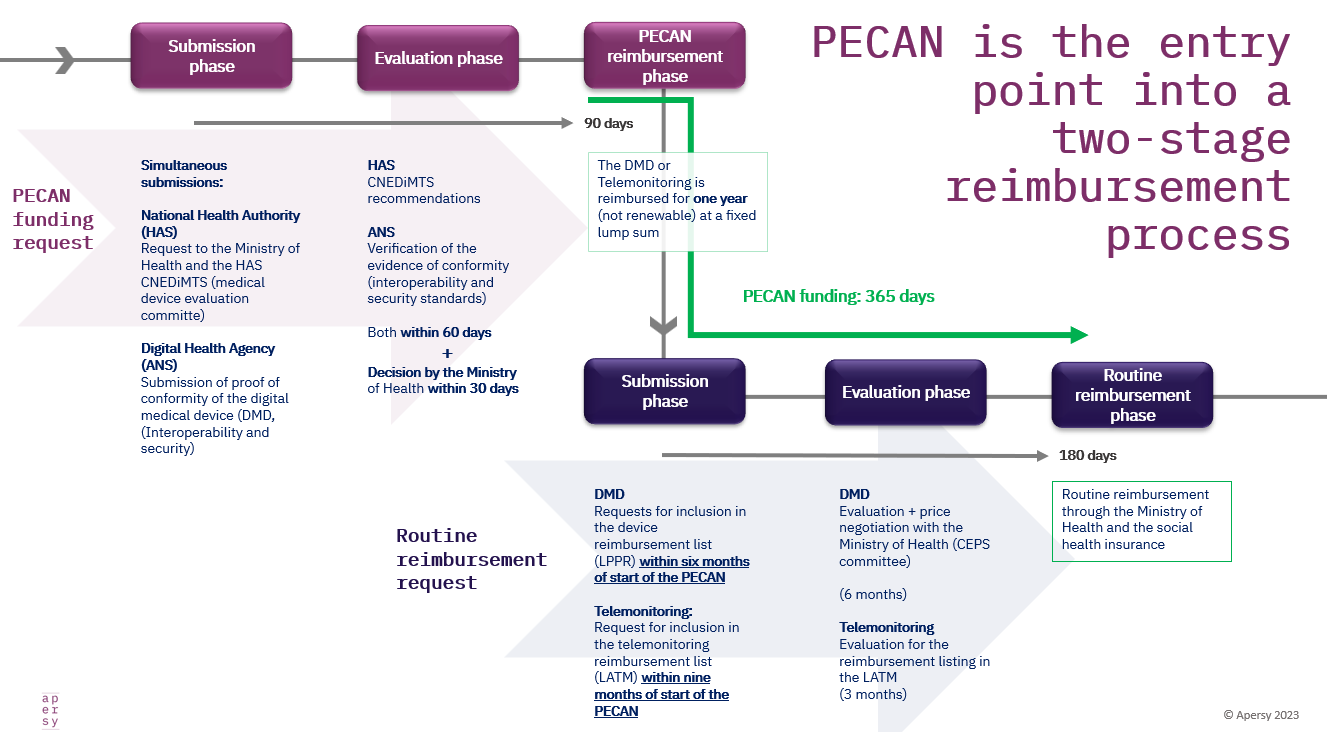
French PECAN reimbursement applications have started… It’s not a DiGA copy-paste
…. the fundamental differences in the French and German access paths for digital medical devices
-

Apersy at the 11th Microbiome & Probiotic R&D & Business Collaboration Forum: Europe, Rotterdam, 23-24 May 2023
Apersy puts focus on market access preparedness at the 11th Microbiome & Probiotic R&D & Business Collaboration Forum: Europe (Rotterdam, 23-24 May 2023).
-

Microbiome-targeting medicines: Commercial launch is moving centre stage
While Europe still lags behind in terms of microbiome medicines approvals, in the UK, we have seen the first Health Technology Assessment (HTA) for microbiota medicines. Let’s have a closer look…
-

March 2023 Monthly Reading List
….What we were reading in March….
-

February 2023 Monthly Reading List
Selected reads from recent press or publications in the disruptive value, commercial and reimbursement space.
-

January 2023 Market Access Reading List
Recent news and articles to think about value and evidence development. This month, the news recap comes as short reading lists.
-

December 2022 Market Access news recap
The monthy 3 minutes of selected bits of news: 62 seconds on DiPAs – digital care and nursing applications are launching in Germany Microbiome product Rebyota is now approved in the US – we are keenly watching out for access, uptake and reimbursement learnings… AIFA reimbursement committees merge Germany rare disease medicine Brineura to recevie…
-

November Market Access news recap
November market access news recap For the November recap a few news snippets from the pharma and digital health world: RWE collection in Germany, the Hemgenix gene therapy, early value assessment in England as well as the dynamic French digital health policy landscape. … as usual, three minutes of your time …